Warning: syntax error, unexpected $end, expecting ']' in /www_root/admin/lib/../php_browscap.ini on line 59 in /www_root/admin/lib/commonlib.php on line 824
Warning: Invalid argument supplied for foreach() in /www_root/admin/lib/commonlib.php on line 826
- K-REACH
- K-BPR
- Product Consulting
-
CCA
 CCA(Chemical Control Act) and IPPC (Integrated Pollution Prevention and Control Act)
CCA(Chemical Control Act) and IPPC (Integrated Pollution Prevention and Control Act) - Risk Assessment
-
Overseas Registration
 Overseas
Overseas
Registration - GHS MSDS
-
IT Solution
-
Resources
-
Company Profile
Active Substance Approval
For existing active substance, a notification is required to obtain a grace period. After the notification, the approval shall be obtained sequentially by product type. For new active substance, it must get approved before manufacture and import regardless of the grace period.
Notification of existing active substance
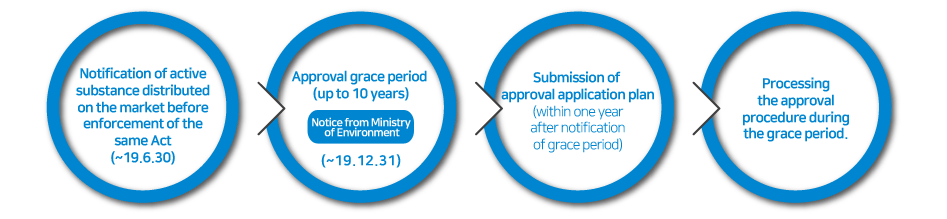
Any person who intends to manufacture or import an existing active substance which was contained in biocidal products distributed domestically until 31st December 2018 will be required to make a notification within 1st January 2019 to 30th June 2019 to obtain a grace period of substance approval. When notifying the active substance, the product type of the substance must be declared together and a grace period of up to 10 years may be granted depending on the product type. During this period, it’s allowed to manufacture and import notified existing active substance without approval.
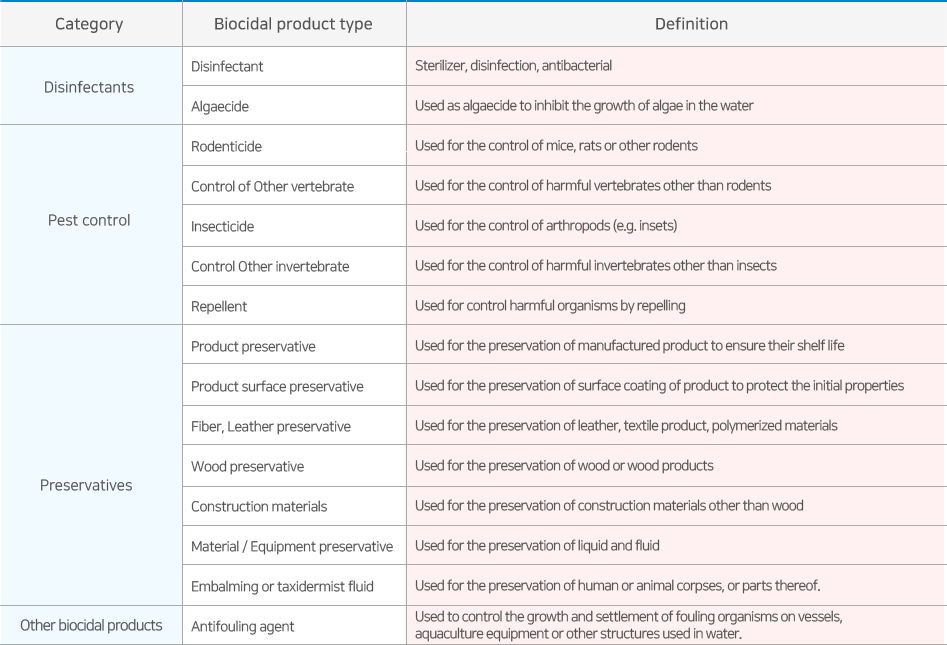
The products containing the active substances are classified into the following 15 types as follows according to the Korean Chemical Safety Act.
Application for substance approval
A person who has notified existing active substance must submit a plan for substance approval application (a proposal on the procedure and method of preparing the substance approval application document) within one year from the date the notified substance is designated as the active substance. Those who submit a substance approval application plan must prepare the appropriate materials accordingly.
Approval grace period according to existing active substance’s biocidal product type
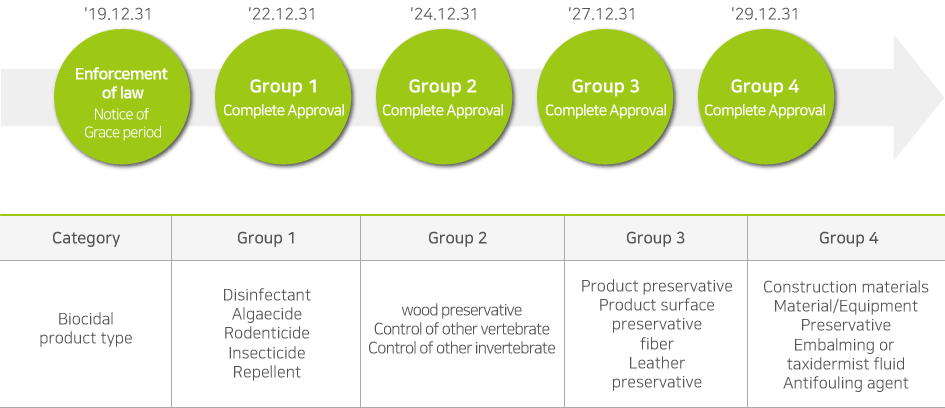
※ Can be adjusted by taking into consideration of the risk, the manufacturing/importing quantity and its regulatory status
Active substance approval
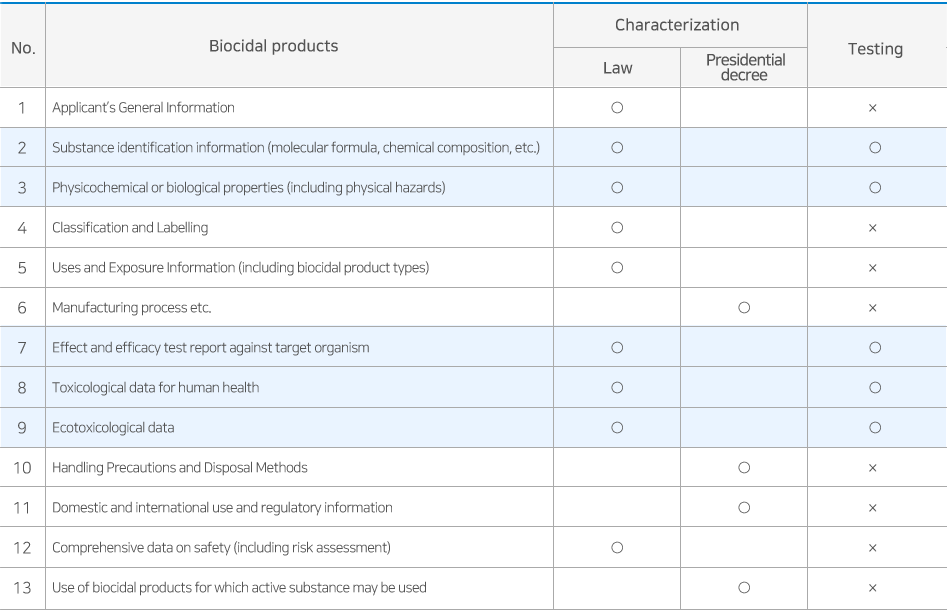
Any person who intends to manufacture or import active substance for use in biocidal products must prepare and submit the appropriate data and obtain approval from the Minister of the Environment. The validity of active substance approval is set within 10 years depending on the properties of the substance and must be re-approved before expiration.
Chemtopia consults as follows
- · Notification of existing active substance
- · Compilation and preparation of substance's equivalency data for the active substance
- · Active substance approval
- · SIEF management
- · Data gap analysis and communication with overseas data owners
- · Preparation of risk assessment
- · Required test arrangement and scheduling
- · Response to the client's inquiry about Regulatory compliance







